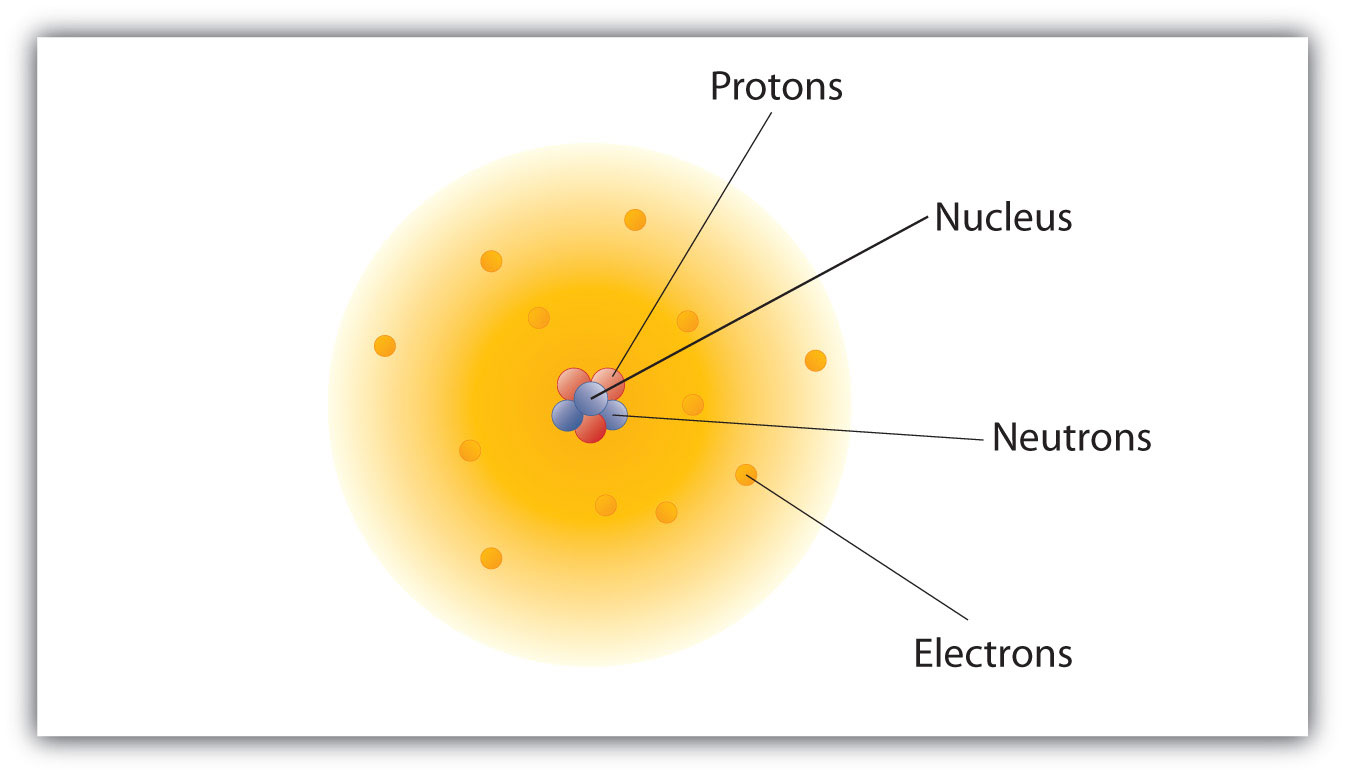
Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells The numbers of subatomic particles in an atom can be calculated from itsAug 15, · The Bohr model shows the three basic subatomic particles in a simple manner Most of an atom's mass is in the nucleus— a small, dense area at the center of every atom, composed of nucleons Nucleons include protons and neutrons All the positive charge of an atom is contained in the nucleus, and originates from the protonsJun 30, 08 · 5 Atoms Molecules Ions Particle Mass (kg) Mass (amu) Charge Electron x 10 31 1 Proton x 10 27 1 Neutron x 10 27 0 Most of the mass in an atom is in the nucleus Subatomic Particles 6

Thomson S Atomic Model Ck 12 Foundation
Model of an atom and its subatomic particles
Model of an atom and its subatomic particles-In this video we cover the structure of atoms, what are subatomic particles, energy levels, and stable and reactive atomsTranscript and notesAtomic structurMay 06, 19 · The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged) Protons and neutrons form the atomic nucleus Electrons are attracted to the protons in the nucleus, but are moving so quickly they fall toward it (orbit) rather than stick to protons




The Discovery Of The Structure Of The Atom And Its Subatomic Particles Studocu
They will also look at an atom and its cation as well as an atom and its anion **This video has no audio** Periodic Table, Model of the Atom, Quantum Numbers, Electron Configuration, Subatomic Particles, Electrons High SchoolNov 30, · Sufficient electrons surround the nucleus This atomic model was discovered through the bombardment experiment of alpha particles on gold foil The electrons in an atom move in shells around the nucleus which contains protons James Chadwick proved the existence of neutrons, the neutral particles in the nucleus(2nd diagram of the atom) Discovered the electron Performed the cathode ray experiment and determined that the ray was composed of negative particles, called electrons, determine the mass charge ratio for an electron and came up with the "plum pudding model" of the atom
The number of subatomic particles in an atom can be calculated from the atom's atomic number and mass number In this model, the atom is a ball of positive charge with negative electronsAre you struggling with organic chemistry?Mar 30, 10 · These particles were electrically neutral and called neutrons With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom They could explain that an atom is made up of electrons, neutrons, and protons The center of an atom is the nucleus that contain protons and neutrons
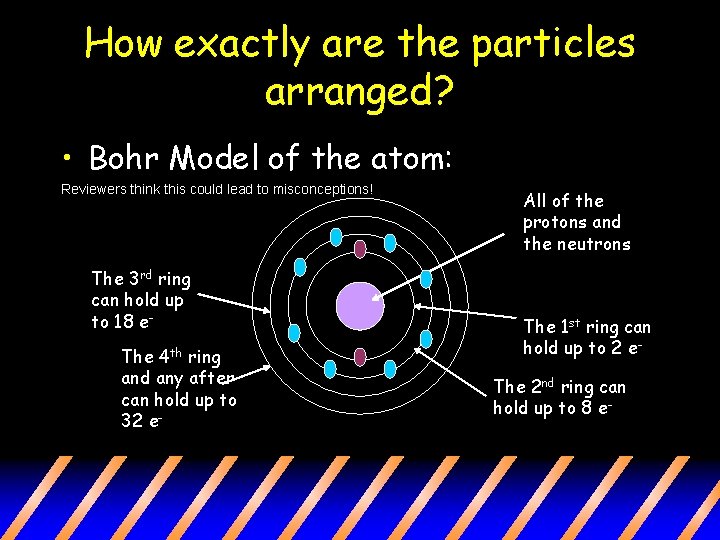
Mar 25, · Correctly construct and interpret a Bohr model for an atom Describe the structure of an atom including its mass, its electrical charge, and subatomic particles Determine the quantity of electrons, protons and neutrons in an atom, given essential information Identify that protons determine an element's identity Chemistry TopicsSep 09, 17 · An excited electron can fall back to its original orbit by emitting energy as radiation;Answer Atoms are extremely small, typically around 100 picometers across Under most definitions, the radii of isolated neutral atoms range between 30 and 300 pm (trillionths of a meter) 2 What are the particles of an atom?




What Is The Lightest Subatomic Particle Qna




Subatomic Particle Subatomic Particle Helium Atom Chemical Element Science Chemical Element Sphere Particle Png Pngwing
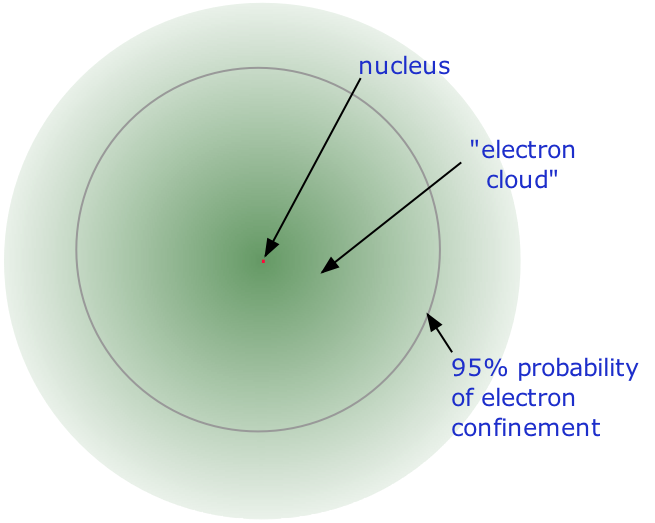
The Bohr model refers to the treatment of electrons as particles that orbit the nucleus Quantum Mechanics The term quantum mechanics refers to energy levels and the theoretical area of physics and chemistry where mathematics is used to explain the behaviour of subatomic particlesThe observation that an atom has slightly less mass than the sum of the masses of its subatomic particles is called which of the following?Jun 17, 15 · The Bohr model is outdated, but it depicts the three basic subatomic particles in a comprehensible way Electron clouds are more accurate representations of where electrons are found Darker areas represent where the electrons are more likely to be found, and lighter areas represent where they are less likely to be found
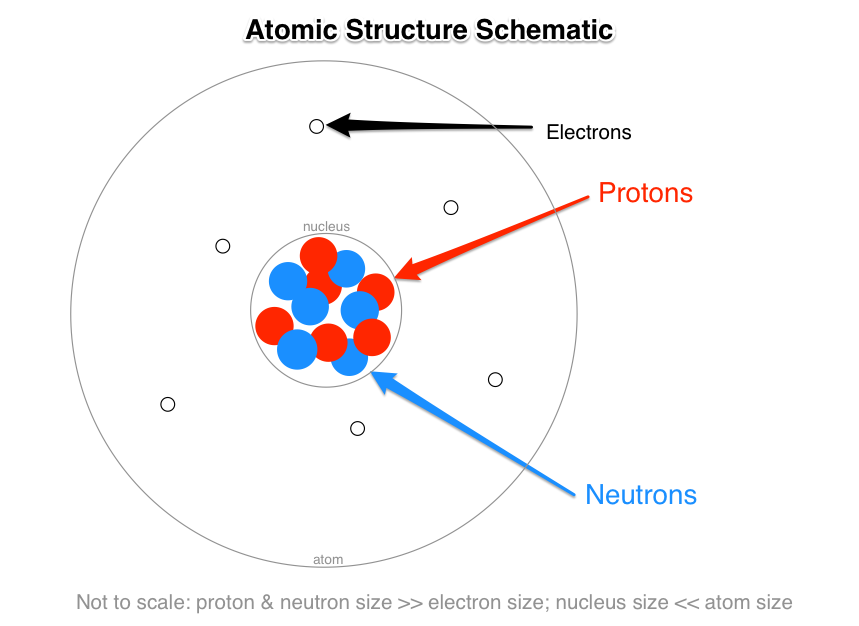



What Are The Names Charges And Locations Of The Three Types Of Subatomic Particles That Make Up An Atom Socratic




Subatomic Particle Definition Examples Classes Britannica
Neutron Neutral Particles 12 A neutron is a subatomic particle found in the nucleus of every atom except that of simple hydrogen The particle derives its name from the fact that it has no electrical charge;Jan 21, · The three main subatomic particles that form an atom are protons, neutrons, and electrons The center of the atom is called the nucleus First, let's learn a bit about protons and neutrons, and then we will talk about electrons a little later Protons and neutrons make up the nucleus of an atom Also, what atomic particle in an atom is the same for each element?Feb 01, 19 · A subatomic particle is defined as a particle that is smaller in magnitude than an atom The list of subatomic particles has been growing steadily since the discovery of the electron in the late 1800s resulting in the need for new fields of study




Bohr Model Description Development Britannica
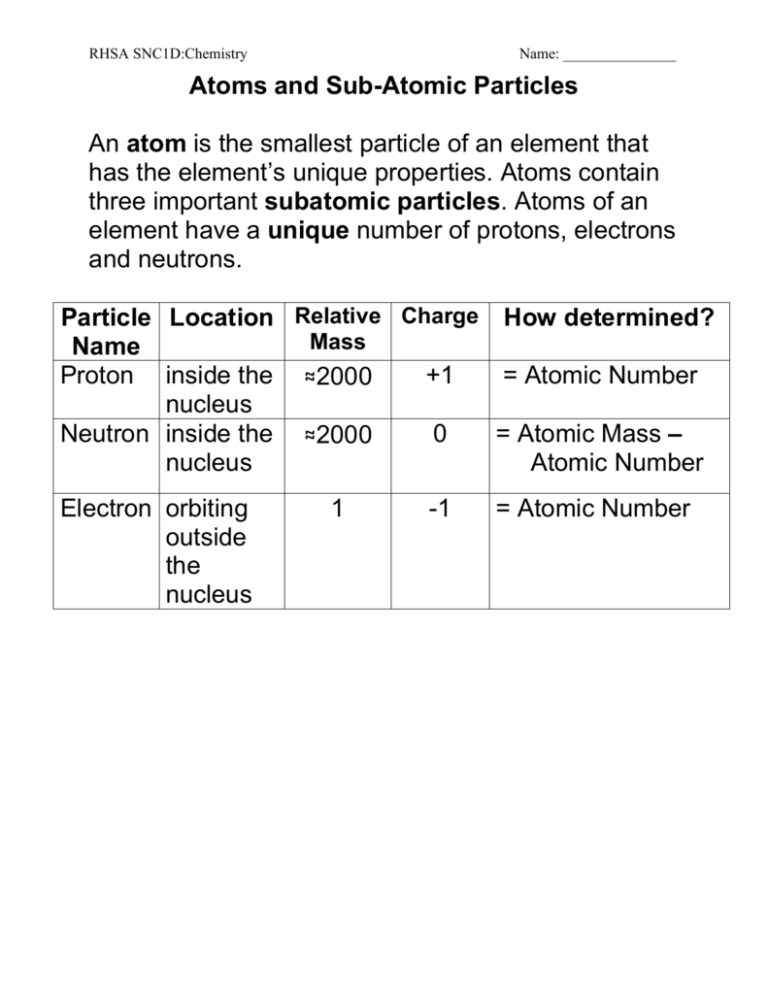



09 Subatomic Particles And Models Of The Atom
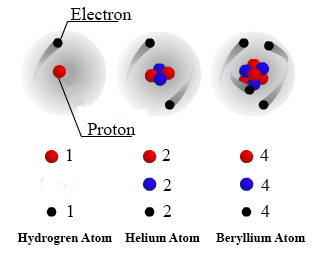
The word 'atom' comes from the Greek word 'atomio' which means 'uncuttable' or 'nondivisible'Scientists believed that atoms were indivisible for the longest time However, in the early th century, some scientists showed that atoms can be further divided into smaller parts such as electrons, protons, and neutronsThese are called subatomic particlesIs that electric fields have been totally eliminated from an atomic structure Properties of atLater, the scientists discovered particles inside the atom that proved, the atoms are divisible The discovery of particles inside atoms led to a better understanding of chemical species, these particles inside the atoms are called subatomic particles The discovery of various subatomic particles is as follows Thomson Atomic Model
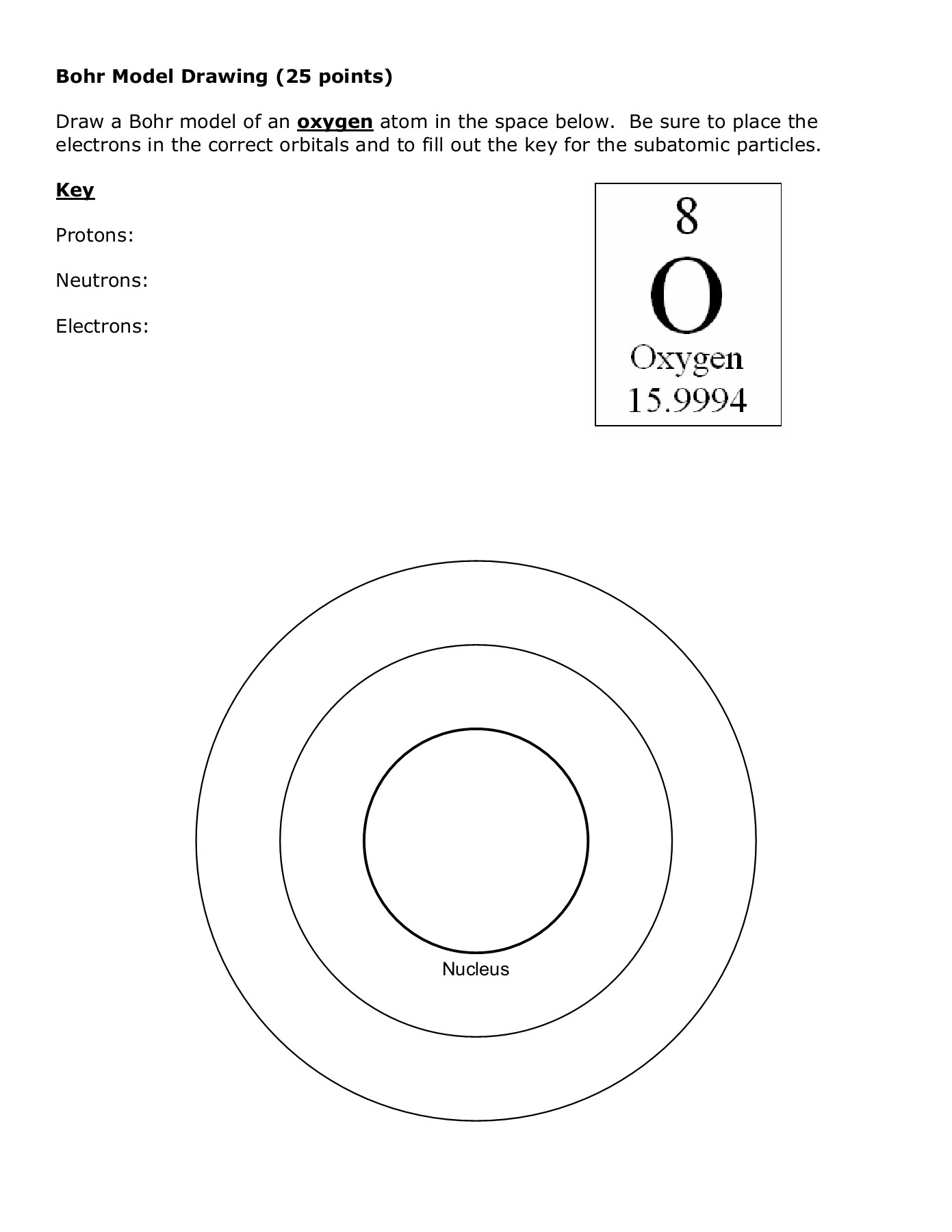



Atomic Structure Worksheet Shelby County Schools Flip Ebook Pages 1 4 Anyflip Anyflip
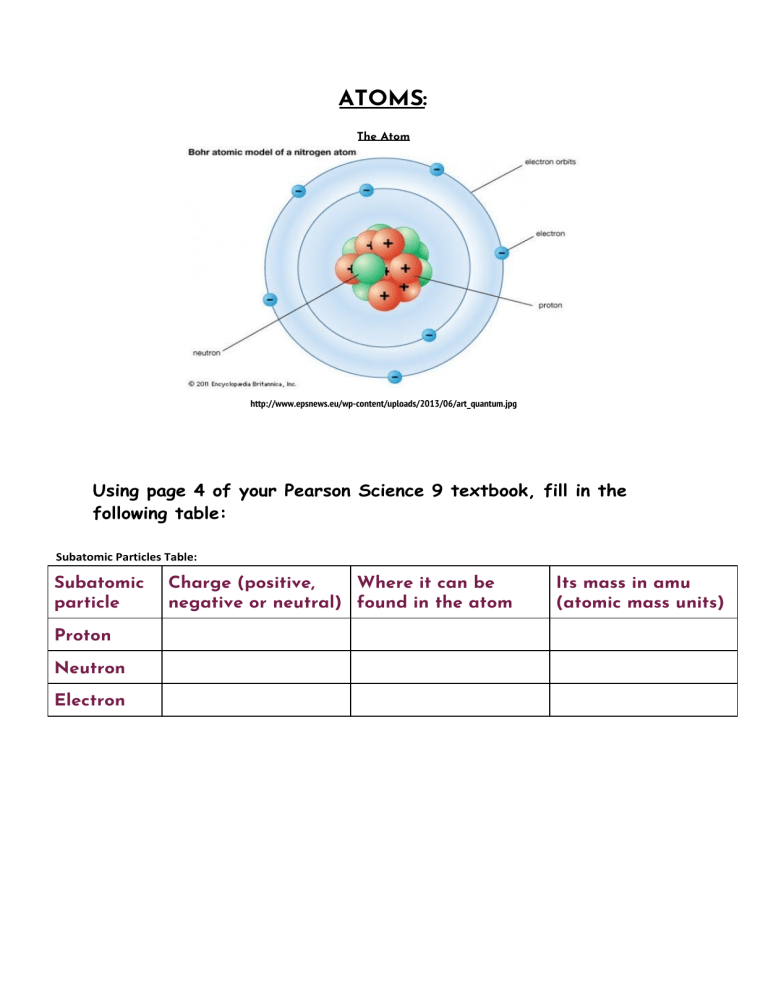



What Is Subatomic Particles In Chemistry
4 Electrons can only exist in certain discrete energy levels 22 James Chadwick Model According to him, the nucleus of an atom contains neutrons, electrically neutral particles with a mass similar to that of proton 23It is neutral electron Negatively Charged 11 An electron is a negatively charged subatomic particle It can be either free (not attached to any atom) or bound to the nucleus of an atomThe modern model of the atom teaches us that all atoms are made up of subatomic particles Subatomic means 'smaller than the atom' In the next section, we are going to learn more about these interesting little particles



Atomic Theory Body Used Process Law Chemical Form Energy Parts Effects




Q5 Draw A Neat Labelled Diagram Representing An Atom Name Th Lido
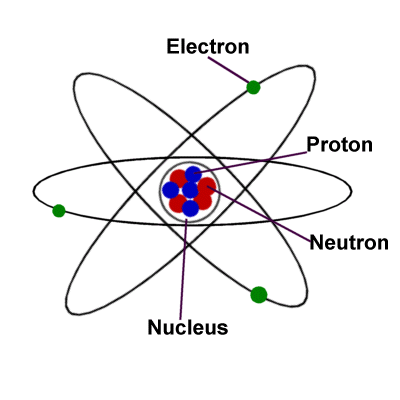
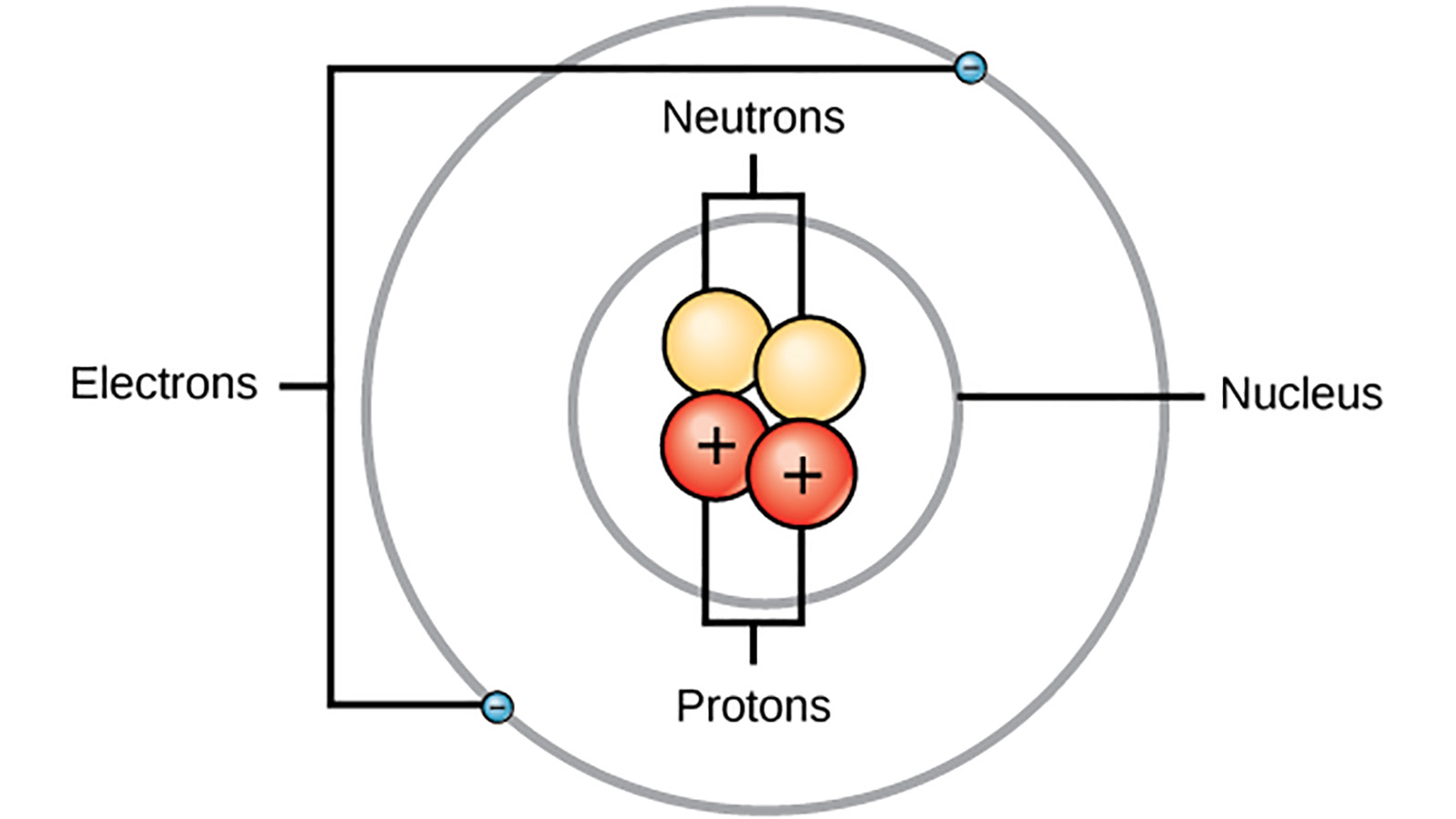
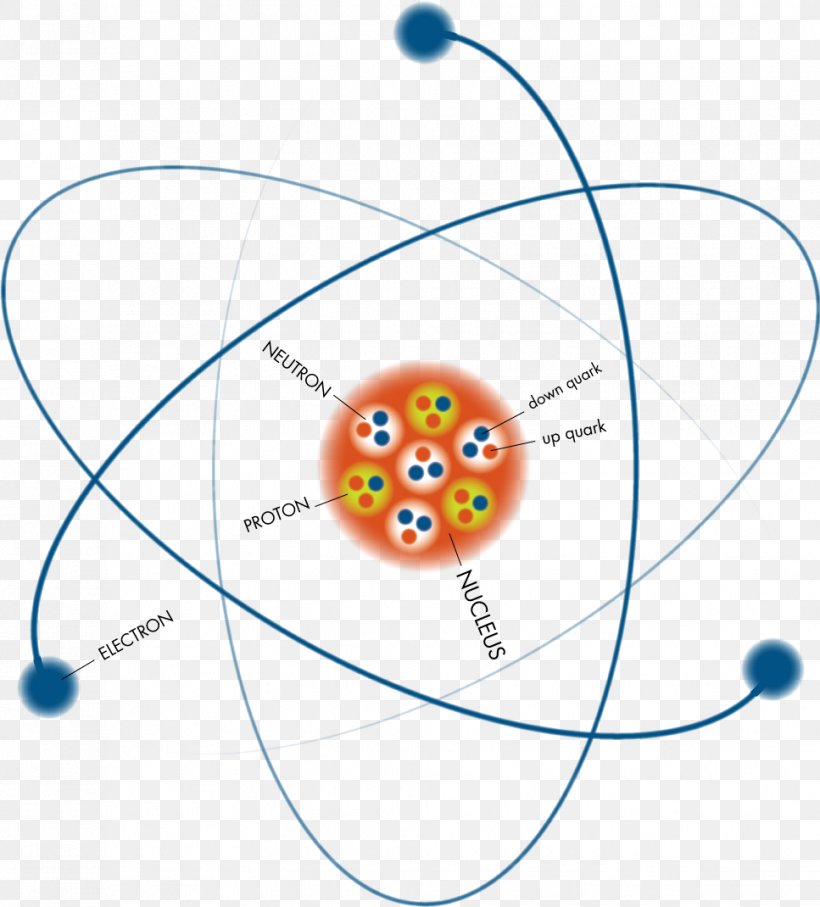
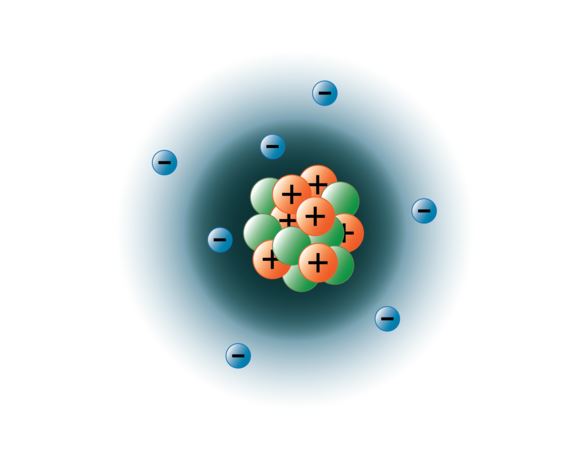
Atoms are made up of three smaller, or subatomic, particles The electrons are spread out around the edge of the atom They orbit the nucleus in layers called shells (energy levels) The protons and neutrons exist in a dense core at the centre of the atom This is called the nucleus The radius of a nucleus is less than 1/10,000 of that of theAug 26, 17 · The subatomic particles are protons, neutrons and electrons And they are related to one another by their mass and charge The properties of these particles are summarized in the table below In nature, an important rule is that positive charged particles always attract negative charged particles, and negative charged particles always repelThe structure of an atom can only be derived, if electric fields are incorporated as part if that structure The failure of current models of the atom;




Introduction To The Bohr Atom Model 1 Introduction



Science For Kids The Atom
The discovery of subatomic particles led to the search how the subatomic particles are arranged in an atom JJ Thomson was the first and one of the many scientists who proposed models for the structure of an atom JJ Thomson discovered negatively charged particles by cathode ray tube experiment in the year 17Subatomic Particles Lesson Plan At the beginning of class, state the goals for the day The goals for day 1 of this lesson are Students will be able to explain and model the different parts of an atom Students will be able to know how many electrons, protons, and neutrons there are based on the element symbolAn atom is the smallest unit of ordinary matter that forms a chemical elementEvery solid, liquid, gas, and plasma is composed of neutral or ionized atoms Atoms are extremely small, typically around 100 picometers across They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects




Elements And Atoms The Building Blocks Of Matter Anatomy And Physiology I



The Bohr Model Quickly Replaced But Never Forgotten Howstuffworks
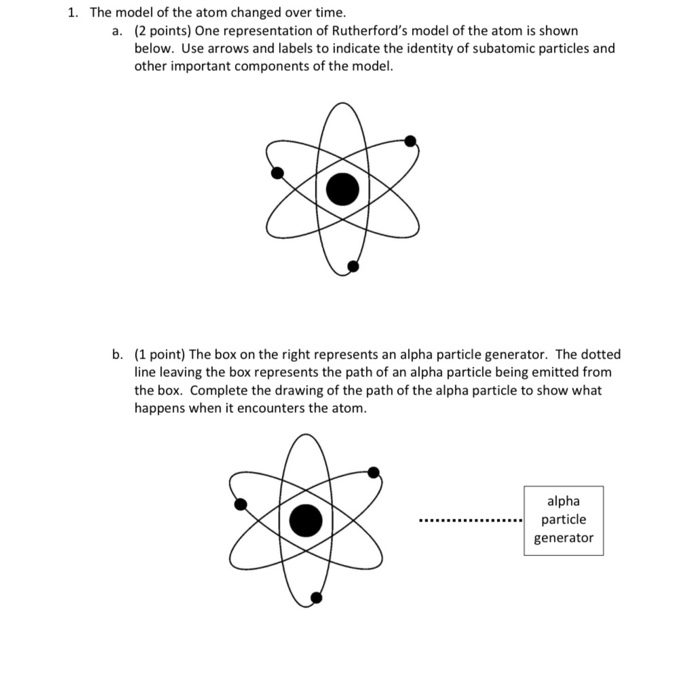
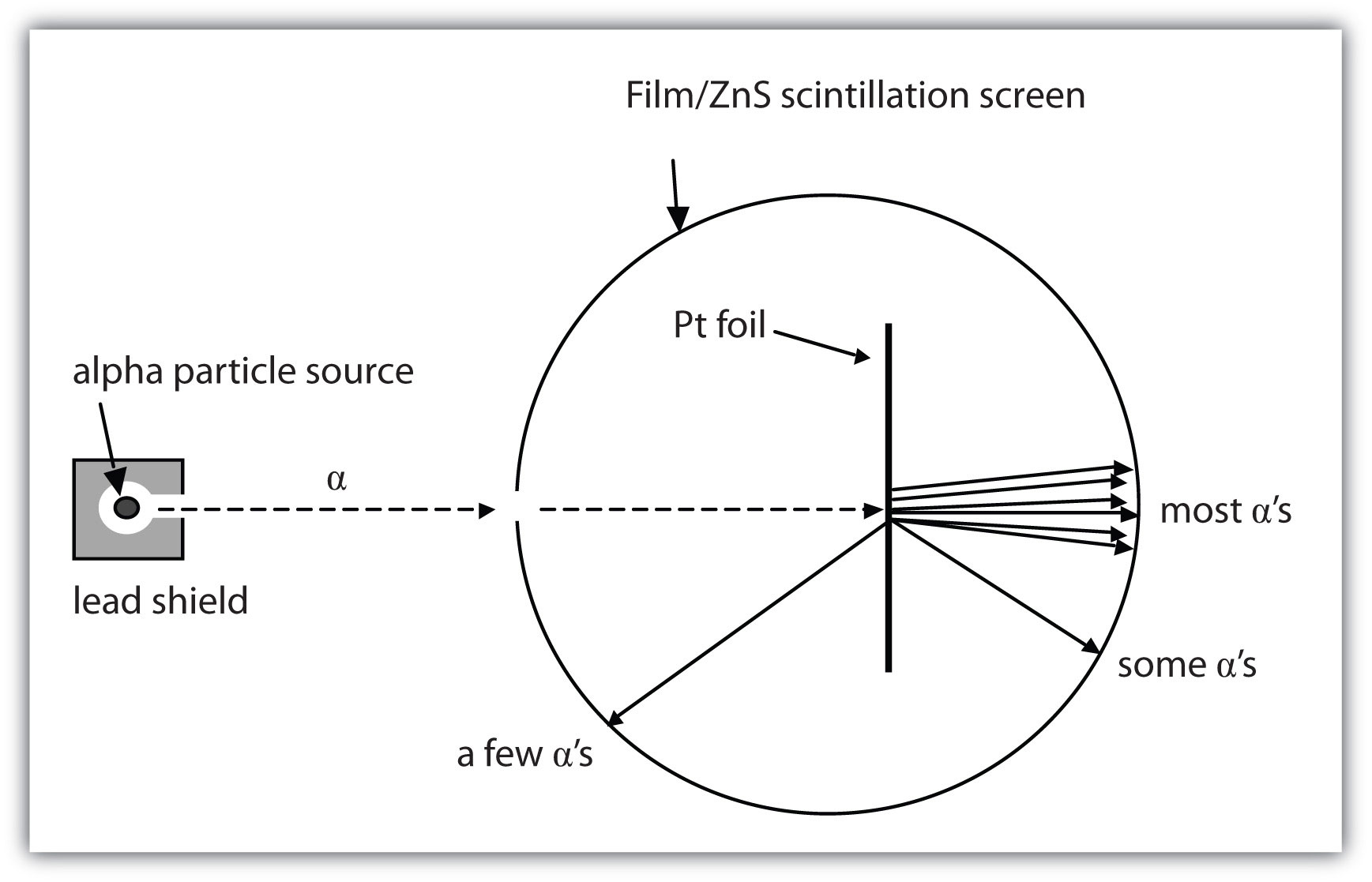
Mass defect mass discrepancy law of conservation of matter law of conservation of energy The model for the atom that was understood when Bohr made his contributions was called the planetary model plumPredict the paths taken by α particles that are fired at atoms with a Rutherford atom model structure Explain why you expect the α particles to take these paths If α particles of higher energy than those in (a) are fired at Rutherford atoms, predict how their paths will differ from the lowerenergy α particle pathsSep 24, 15 · Table 1 shows the relative mass and charge of subatomic particles Protons are positively charged and electrons are negatively charged while neutrons have no charge An atom has same number of protons and electrons so the atom is electrically neutral A proton has same mass as a neutron Table 1 shows charges and masses of subatomic particles



Nondestructive Evaluation Physics Atomic Elements



Difference Between Bohr And Rutherford S Atomic Models With Comparison Chart Bio Differences
Aug 25, · Atoms consist of electrons, protons, and neutrons Although this is an oversimplification that ignores the other subatomic particles that have been discovered, it is sufficient for discussion of chemical principles Some properties of these subatomic particles are summarized in Table \(\PageIndex{1}\), which illustrates three important pointsApr 30, · During this explosion the first subatomic particles that make up matter and energy were createdAt 10^11 seconds the weak nuclear force split form the others, allowing formation of the first quarks, the building blocks of subatomic particlesLater on, at 10^4 seconds the first protons and neutrons were formedSubatomic Particles and the Nuclear Atom Section 42 Subatomic Particles and the Nuclear Atom Discovery of the Atom and its Particles Dalton s Model In the early 1800s, the English Chemist John Dalton PowerPoint PPT presentation free to view
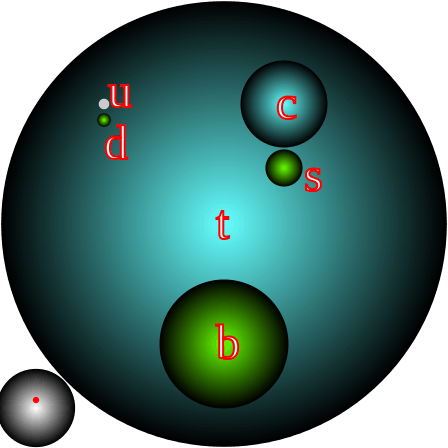



What Are The Parts Of An Atom



Atoms Course Hero
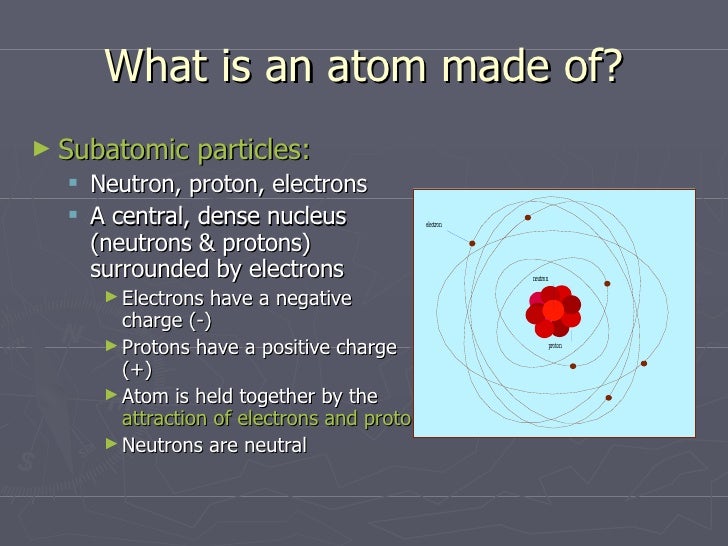
Atoms consist of three basic particles protons, electrons, and neutrons The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge) The outermost regions of the atom are called electron shells and contain the electrons (negatively charged)Rutherford's model is essentially the same model that we use today to describe atoms but with one important modification The planetary model suggests that electrons occupy certain specific, circular orbits about the nucleus We know now that this model is overly simplistic A better description is that electrons form fuzzy clouds around nucleiApr 30, 21 · Students use gumdrops and toothpicks to make lithium atom models Using these models, they investigate the makeup of atoms, including their relative size Students are then asked to form molecules out of atoms, much in the same way they constructed atoms out of the particles that atoms are made of Students also practice adding and subtracting electrons from an atom
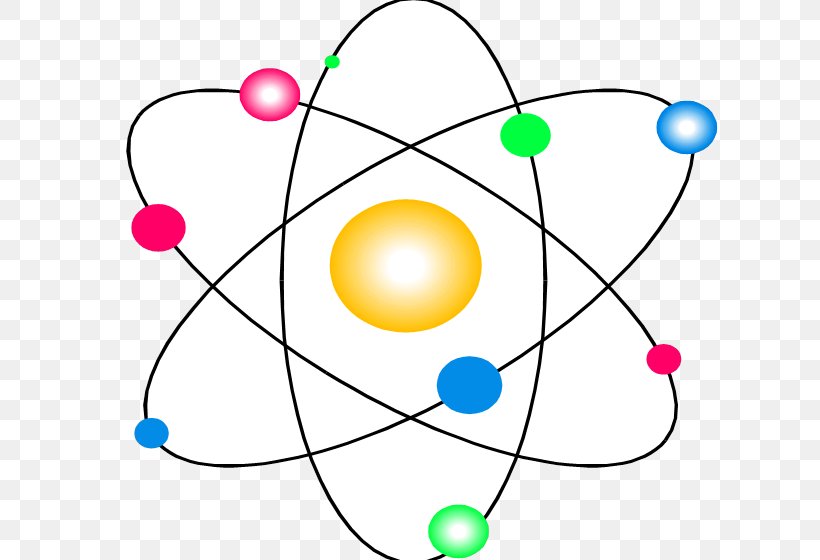



Bohr Model Atomic Theory Subatomic Particle Science Png 624x560px Bohr Model Area Atom Atomic Nucleus Atomic
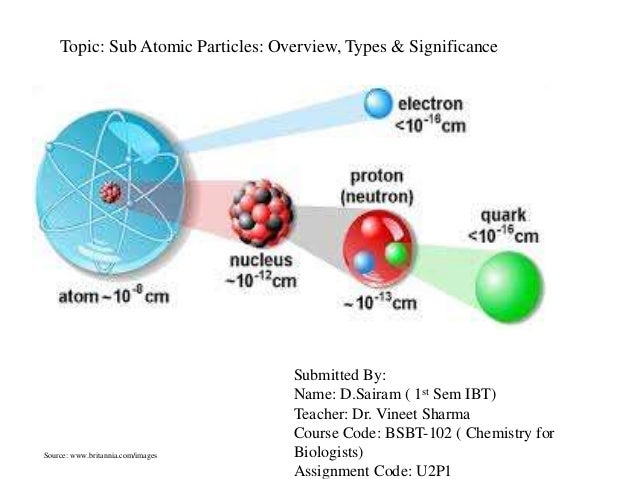



Sub Atomic Particles
Jul 27, 17 · Atoms are made up of three subatomic particles, electrons, protons and neutrons At the centre of an atom proton and neutrons are present, whereas outside theAtomic Structure Activity 4a 1 How small is an atom?According to Standard Model and the concept of quantum vacuum fields, the electric charge of subatomic particles and antiparticles is an innate property of matter Is this is a simple and reasonable answer to the question, given that the charge co



Atomic Models Learn Chemistry Class 9 Amrita Vidyalayam Elearning Network




Untitled Document
Download my free ebook "10 Secrets To Acing Organic Chemistry" here http//leah4scicom/orgoebook/http//leah4scJun 02, · The Standard Model Based on their knowledge of subatomic particles, scientists have developed a theory called the standard model to explain all the matter in the universe and how it is held together The model includes only the fundamental particles in the Table above No other particles are needed to explain all kinds of matterIntroduction to the atom Transcript Learn how atoms are made up of protons, neutrons, and electrons Elements are defined by the atomic number, the number of protons in the nucleus The mass of an atom is determined by the total number of protons and neutrons Created by Sal Khan




Atoms And Atomic Theory Review
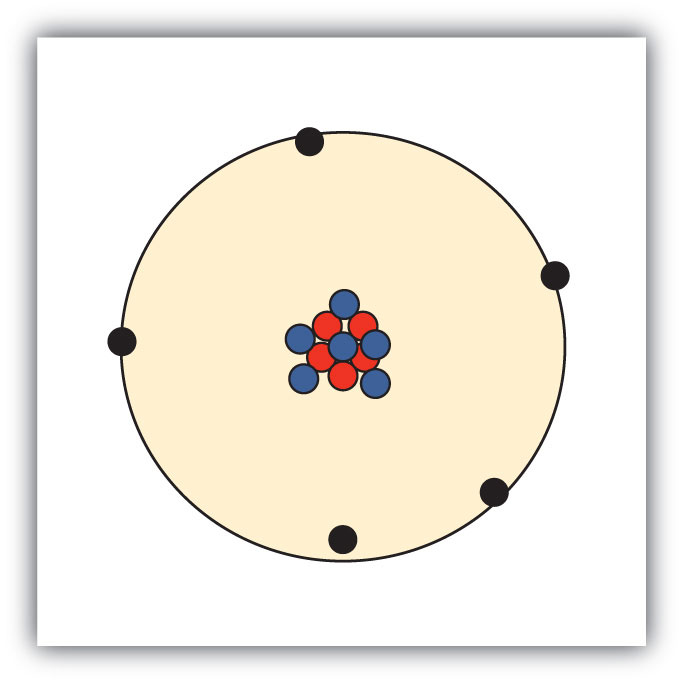



Atomic Theory
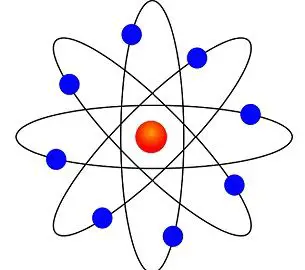
The diagram on the right is traditionally used to represent an atom, as proposed by Niels Bohr in 1913 It was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star) In this model, the electrons can only be at certain energy levelsAnswer Atoms are made up of three particles protons, neutrons, and electrons
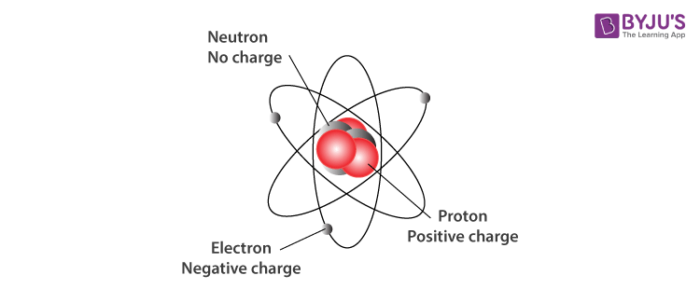



Subatomic Particles Definition Discovery And Key Features




On The Basis Of Rutherford S Model Of An Atom Which Sub Atomic




Sub Atomic Particles Atoms Siyavula



Bohr Model Of The Atom Overview And Examples




Thomson S Atomic Model Ck 12 Foundation




Subatomic Particles Atomic Model Project123
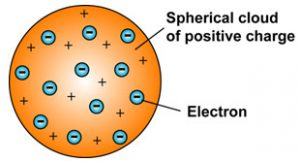



J J Thomson Model Of An Atom Class 9 Structure Of An Atom




Sub Atomic Particles Chemistry Libretexts
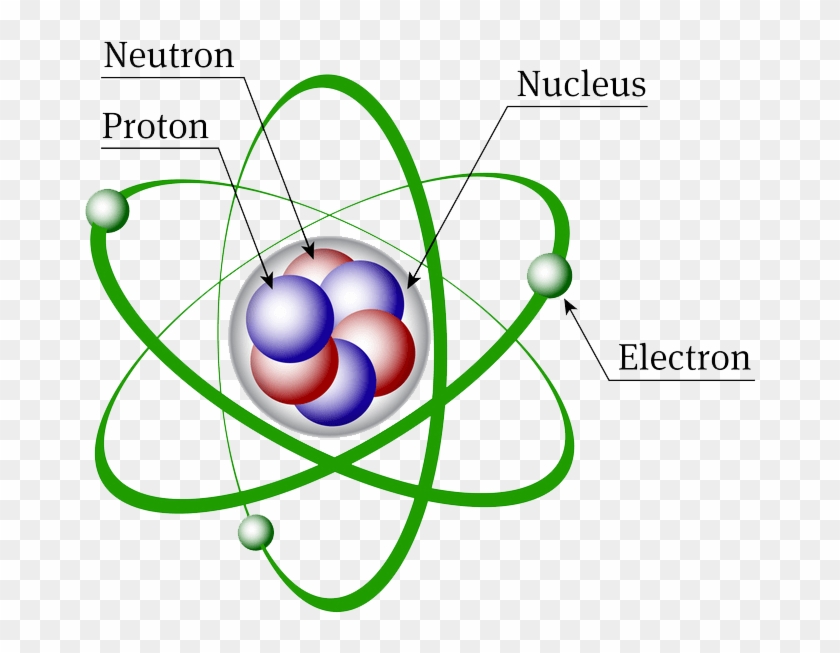



Atomic Structure Discovery Of Subatomic Particles Definition Nuclear Model Of An Atom Free Transparent Png Clipart Images Download
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)



Basic Model Of The Atom Atomic Theory
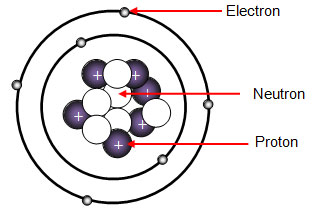



Matter And Energy Atomic Structure Texas Gateway



Subatomic Particle Atomic Nucleus Atomic Physics Png 967x1070px Atom Area Atomic Nucleus Atomic Physics Atomic Theory



Modeling Atoms Pratyusha S Science Notebook




Atoms Lesson Plan A Complete Science Lesson Using The 5e Method Of Instruction Kesler Science



Atomic Structure What Is An Atom Atom The




Rutherford Model Definition Facts Britannica




The Discovery Of The Structure Of The Atom And Its Subatomic Particles Studocu



Atoms And Subatomic Particles



Subatomic Particles




Atomic Structure Electrons Protons Neutrons And Atomic Models




1 01 Subatomic Particles Protons Neutrons Electrons And The Nucleus Week 1 Coursera




Learning Activity 1direction Below Is A Representation Of An Atom Identify And Label The Subatomic Brainly Ph




Discovery Of Subatomic Particle Concept Map Worksheet




Pin By Gen Kelley On School Atoms And Molecules For Kids Science Projects For Kids Atom




Atomic Structure Electrons Protons Neutrons And Atomic Models




Atomic Structure Periodic Table Science Quiz Quizizz



Atomic Structure Subatomic Particles Over The Past Century




Dublin Schools Lesson Isotopes How Do The Number Of Subatomic Particles Differ For Atoms From The Same Element




The Image Below Shows A Model Of The Atom Which Subatomic Particle Does The Arrow In The Image Brainly Com




What Is A Subatomic Particle Definition Mass Video Lesson Transcript Study Com



2 3 The Nuclear Atom Chemistry Libretexts




What Is An Atom Live Science




M M Model Of The Atom Edible Subatomic Particles Elementary Chemistry Atom Model Chemistry Lessons




Compare The Position Of The Subatomic Particles In The Plum Pudding Model With The Nuclear Model Brainly Com



Solved The Model Of The Atom Changed Over Time A One Rep Chegg Com




Introduction To Structure Of Atom Proton Neutron Electron With Examples




The Atom Chemistry Is My Jam Atom Neutrons Electron Configuration



Subatomic Particle Disintegration Violates The Standard Model Of Physics Completely Unexpected




Chapter 5 Atomic Structure Draw And Label The Model Of An Atom What Are The Characteristics That Make The Atom Found In One Substance Different Ppt Download



2 2 2 The Modern Atomic Model Revision My
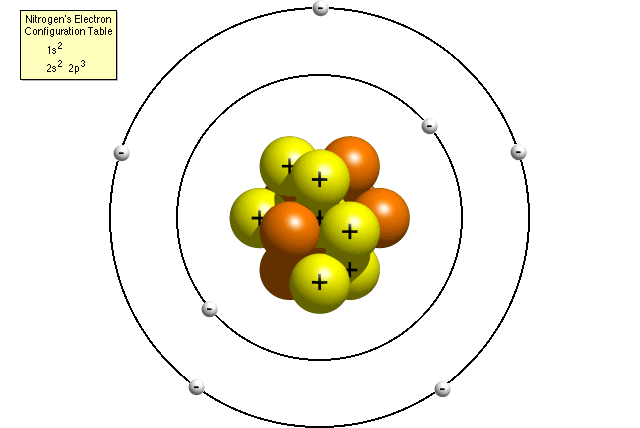



Questions And Answers How Do I Make A Model Of An Atom




Atomic Structure And Subatomic Particles Youtube



The Atom Contains 3 Subatomic Particles Ppt Video Online Download




Subatomic Particles Universe Today




Atom Wikipedia




What Is A Subatomic Particle Definition Mass Video Lesson Transcript Study Com
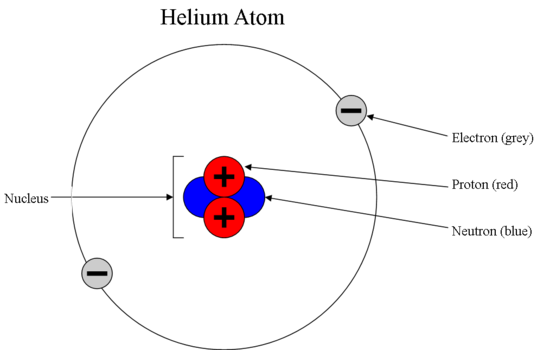



Sub Atomic Particles Chemistry Libretexts
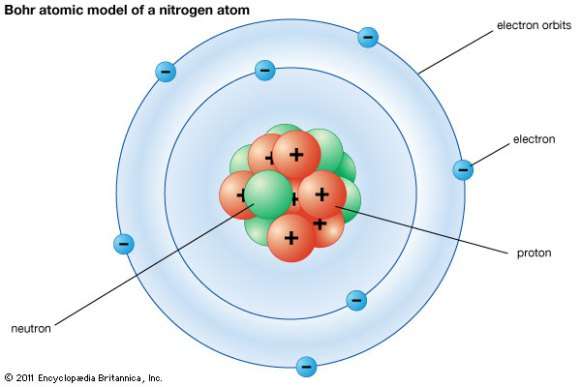



What Are The Parts Of An Atom



The Structure Of Atoms
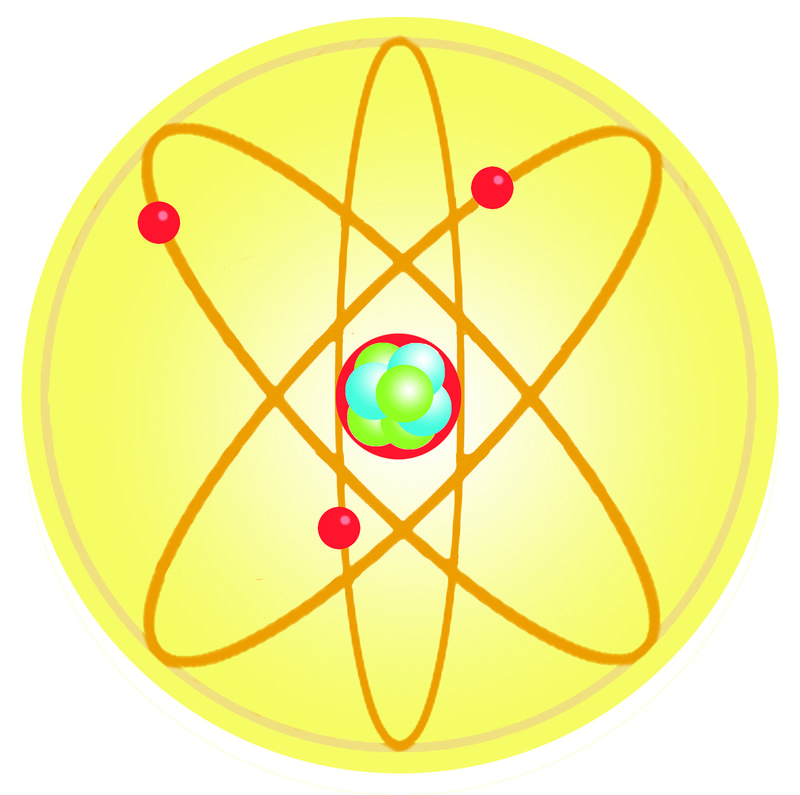



Dalton S Atomic Theory Read Chemistry Ck 12 Foundation



Subatomic Particle Wikipedia




Identification




Atom Symbol Symbol Area Vector Model Of The Atom Subatomic Particle Png Pngwing




The History Of The Atom Theories And Models Compound Interest




The Structure Of Atoms




2 1 Elements And Atoms The Building Blocks Of Matter Anatomy Physiology




Chem 103 The Atom And Its Subatomic Particles
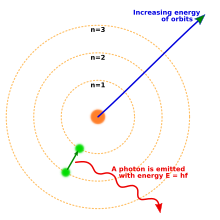



Electron Wikipedia




Lithium Atom Bohr Model Atomic Number Particle Chemical Atom Chemical Element Symmetry Png Pngegg




Atomic Structure Objectives Classify Subatomic Particles Describe The Structure Of An Atom According To Modern Theory Describe How The Atomic Model Ppt Download




Atomic Basics Flashcards Quizlet




What Is A Subatomic Particle Definition Mass Video Lesson Transcript Study Com




History Of The Atom Ck 12 Foundation
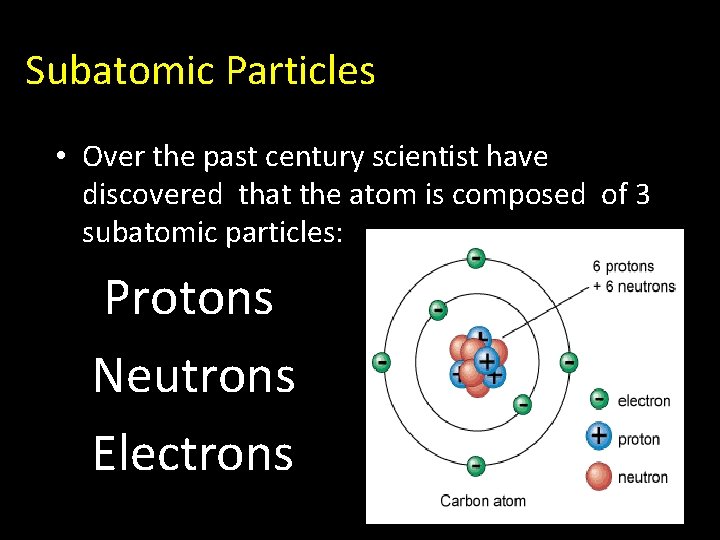



Atomic Structure Subatomic Particles Over The Past Century
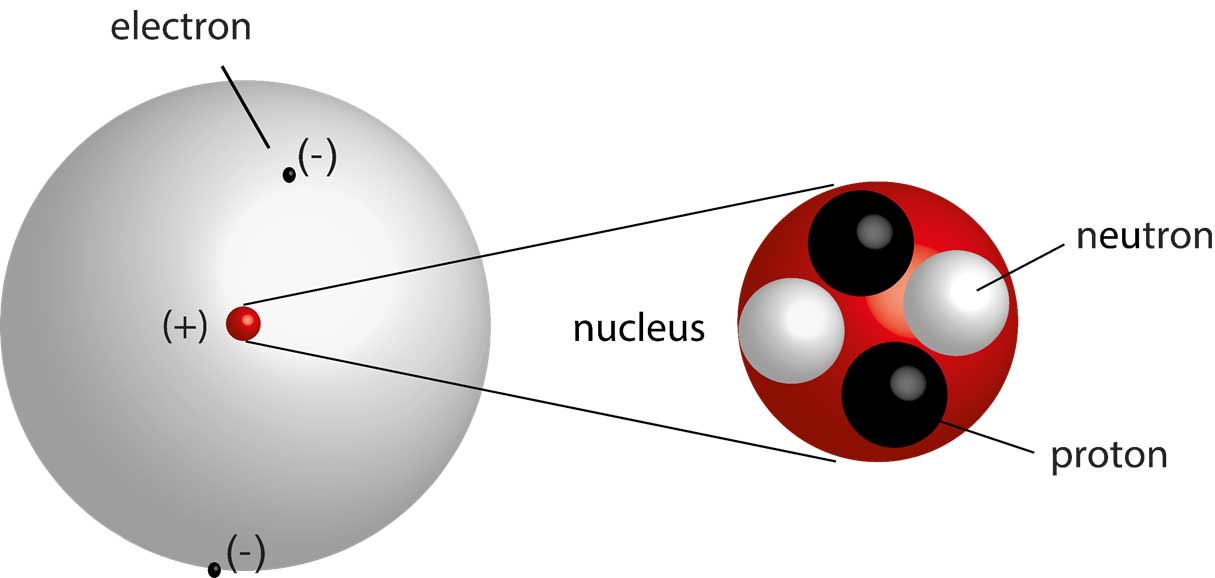



Modern Atomic Theory Be Prepared Everything You Should Know For 1st Year Chemistry



Atomic Theory
/atom-drawn-by-scientist-or-student-155287893-584ee6855f9b58a8cd2fc8f1.jpg)



Subatomic Particles You Should Know
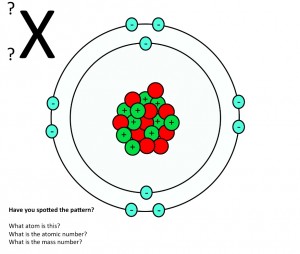



Atomic Structure Teaching Resources The Science Teacher
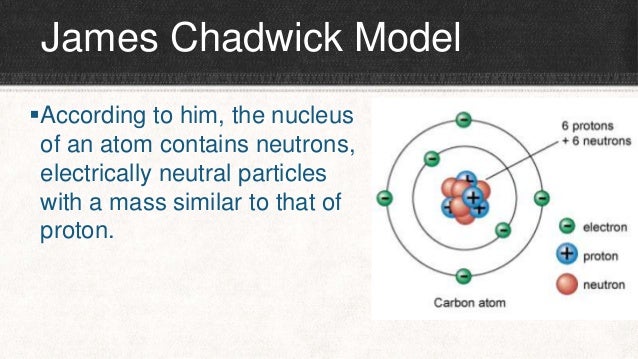



Discovery Of The Structure Of The Atom




Snc1p



The Atom Course Hero




The Image Below Shows A Model Of The Atom Which Subatomic Particle Does The Arrow In The Image Below Brainly Com




No 3198 Subatomic Particles




Atoms Their Subatomic Particles Atoms Chemistry Electrons En Neutrons Particles Protons Science Subatomic Glogster Edu Interactive Multimedia Posters




Solved Tudent Taking Cem 14 We All Term Will Receive Chegg Com
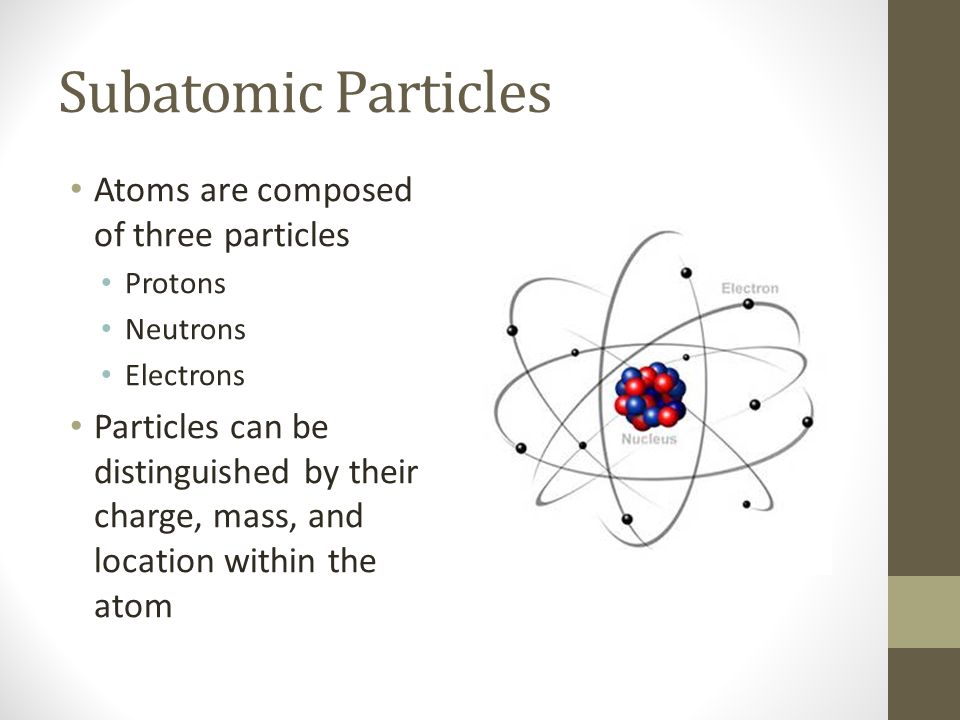



Subatomic Particles Atoms Are Composed Of Three Particles Protons Neutrons Electrons Particles Can Be Distinguished By Their Charge Mass And Location Ppt Download



0 件のコメント:
コメントを投稿